Calorimetry Lab Assumptions . a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. No calorimeter is perfect, however. For example, when an exothermic reaction. A doubled styrofoam cup fitted. For example, when an exothermic reaction. a perfect calorimeter absorbs no heat from the solution that it contains, nor loses any heat to the surroundings. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. You use an aluminum vessel as a calorimeter. the first part of the lab session is devoted to evaluating ccal of your calorimeter. a calorimeter is a device used to determine heat flow during a chemical or physical change. There are two types of calorimetry experiments you need to know. the assumption behind the science of calorimetry is that the energy gained or lost by the water is equal to the energy lost or gained by the object under study.
from www.studocu.com
There are two types of calorimetry experiments you need to know. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. You use an aluminum vessel as a calorimeter. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A doubled styrofoam cup fitted. a perfect calorimeter absorbs no heat from the solution that it contains, nor loses any heat to the surroundings. the first part of the lab session is devoted to evaluating ccal of your calorimeter. a calorimeter is a device used to determine heat flow during a chemical or physical change.
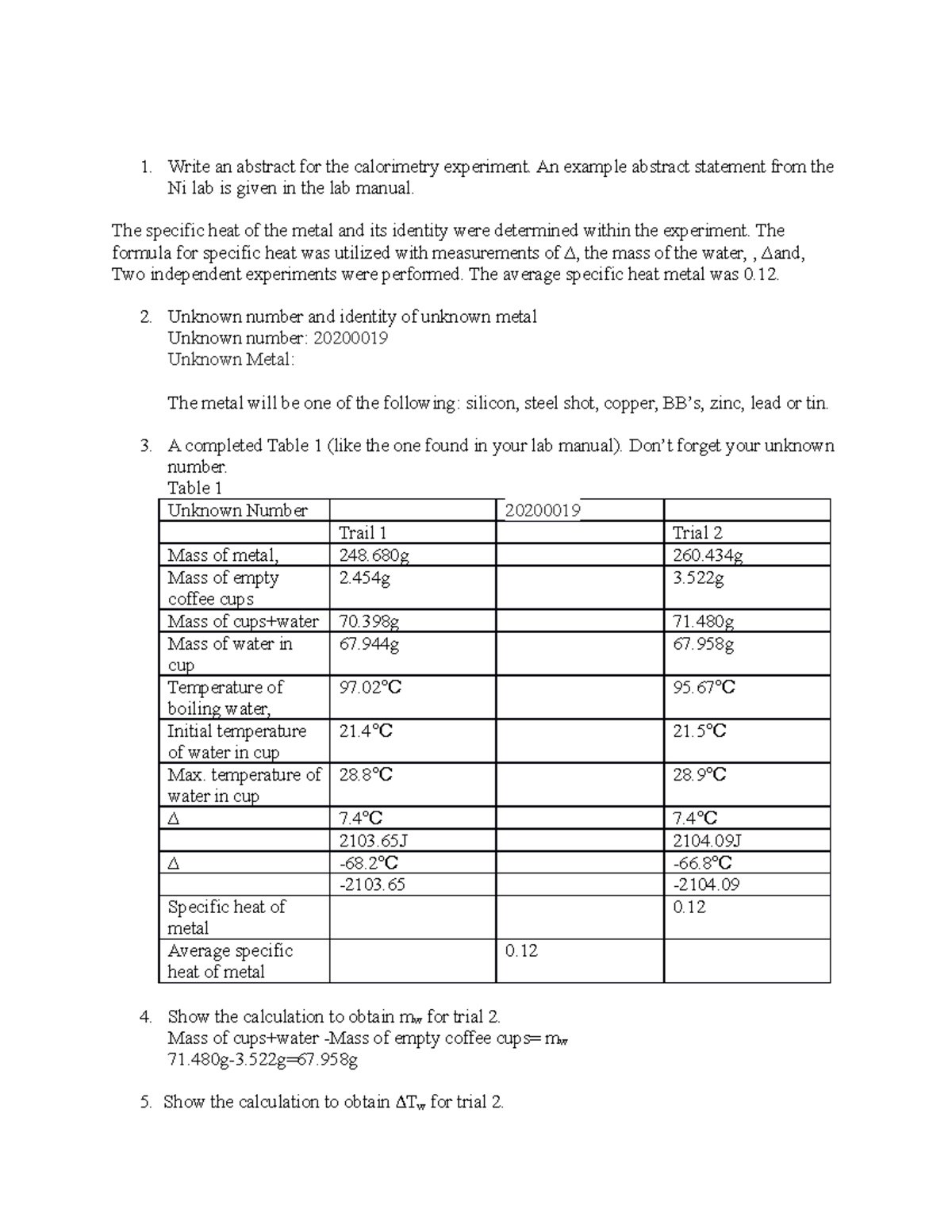
Calorimetry Lab Report Write an abstract for the calorimetry
Calorimetry Lab Assumptions For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to determine heat flow during a chemical or physical change. No calorimeter is perfect, however. A doubled styrofoam cup fitted. a perfect calorimeter absorbs no heat from the solution that it contains, nor loses any heat to the surroundings. For example, when an exothermic reaction. the first part of the lab session is devoted to evaluating ccal of your calorimeter. There are two types of calorimetry experiments you need to know. the assumption behind the science of calorimetry is that the energy gained or lost by the water is equal to the energy lost or gained by the object under study. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. You use an aluminum vessel as a calorimeter. For example, when an exothermic reaction.
From www.studocu.com
Experiment 25 Calorimetry Prelaboratory Assignment CHEM 005 Studocu Calorimetry Lab Assumptions A doubled styrofoam cup fitted. You use an aluminum vessel as a calorimeter. For example, when an exothermic reaction. the first part of the lab session is devoted to evaluating ccal of your calorimeter. There are two types of calorimetry experiments you need to know. a calorimeter is a device used to measure the amount of heat involved. Calorimetry Lab Assumptions.
From stock.adobe.com
Vettoriale Stock illustration of chemistry and physics, Calorimeter Calorimetry Lab Assumptions the assumption behind the science of calorimetry is that the energy gained or lost by the water is equal to the energy lost or gained by the object under study. a calorimeter is a device used to determine heat flow during a chemical or physical change. There are two types of calorimetry experiments you need to know. Web. Calorimetry Lab Assumptions.
From www.animalia-life.club
Calorimeter Diagram Calorimetry Lab Assumptions For example, when an exothermic reaction. the assumption behind the science of calorimetry is that the energy gained or lost by the water is equal to the energy lost or gained by the object under study. a perfect calorimeter absorbs no heat from the solution that it contains, nor loses any heat to the surroundings. a calorimeter. Calorimetry Lab Assumptions.
From studylib.net
Lab 33 calorimetry lab Calorimetry Lab Assumptions the first part of the lab session is devoted to evaluating ccal of your calorimeter. the assumption behind the science of calorimetry is that the energy gained or lost by the water is equal to the energy lost or gained by the object under study. For example, when an exothermic reaction. a calorimeter is a device used. Calorimetry Lab Assumptions.
From www.studocu.com
P calorimetry 25 lab report StuDocu Calorimetry Lab Assumptions the assumption behind the science of calorimetry is that the energy gained or lost by the water is equal to the energy lost or gained by the object under study. a calorimeter is a device used to determine heat flow during a chemical or physical change. For example, when an exothermic reaction. There are two types of calorimetry. Calorimetry Lab Assumptions.
From www.studocu.com
Calorimetrylab compress Calorimetry Lab General Chemistry (Chem 112 Calorimetry Lab Assumptions a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. You use an aluminum vessel as a calorimeter. the assumption behind the science. Calorimetry Lab Assumptions.
From www.studocu.com
Expt25 Calorimetry lab report Expt25 Calorimetry Data A Calorimetry Lab Assumptions A doubled styrofoam cup fitted. a calorimeter is a device used to determine heat flow during a chemical or physical change. For example, when an exothermic reaction. There are two types of calorimetry experiments you need to know. the first part of the lab session is devoted to evaluating ccal of your calorimeter. a calorimeter is a. Calorimetry Lab Assumptions.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Calorimetry Lab Assumptions A doubled styrofoam cup fitted. You use an aluminum vessel as a calorimeter. a calorimeter is a device used to determine heat flow during a chemical or physical change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. No calorimeter is perfect,. Calorimetry Lab Assumptions.
From www.slideserve.com
PPT Molar Enthalpy PowerPoint Presentation, free download ID6624841 Calorimetry Lab Assumptions For example, when an exothermic reaction. the assumption behind the science of calorimetry is that the energy gained or lost by the water is equal to the energy lost or gained by the object under study. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. There are two. Calorimetry Lab Assumptions.
From users.highland.edu
Calorimetry Calorimetry Lab Assumptions For example, when an exothermic reaction. No calorimeter is perfect, however. For example, when an exothermic reaction. a perfect calorimeter absorbs no heat from the solution that it contains, nor loses any heat to the surroundings. the assumption behind the science of calorimetry is that the energy gained or lost by the water is equal to the energy. Calorimetry Lab Assumptions.
From www.scribd.com
Calorimetry Lab Explanation Chemistry Physical Sciences Free 30 Calorimetry Lab Assumptions For example, when an exothermic reaction. A doubled styrofoam cup fitted. a perfect calorimeter absorbs no heat from the solution that it contains, nor loses any heat to the surroundings. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. the assumption behind the science of calorimetry is. Calorimetry Lab Assumptions.
From chemwiki.ucdavis.edu
Chapter 9.6 Calorimetry Chemwiki Calorimetry Lab Assumptions a calorimeter is a device used to determine heat flow during a chemical or physical change. You use an aluminum vessel as a calorimeter. the first part of the lab session is devoted to evaluating ccal of your calorimeter. A doubled styrofoam cup fitted. No calorimeter is perfect, however. For example, when an exothermic reaction. the assumption. Calorimetry Lab Assumptions.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Calorimetry Lab Assumptions A doubled styrofoam cup fitted. the assumption behind the science of calorimetry is that the energy gained or lost by the water is equal to the energy lost or gained by the object under study. a calorimeter is a device used to determine heat flow during a chemical or physical change. There are two types of calorimetry experiments. Calorimetry Lab Assumptions.
From lessonschooluninserted.z14.web.core.windows.net
How To Calculate Enthalpy Chemistry Calorimetry Lab Assumptions A doubled styrofoam cup fitted. the assumption behind the science of calorimetry is that the energy gained or lost by the water is equal to the energy lost or gained by the object under study. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is. Calorimetry Lab Assumptions.
From www.pinterest.com
Calorimetry Lab Report in 2022 Science diagrams, Lab report Calorimetry Lab Assumptions a perfect calorimeter absorbs no heat from the solution that it contains, nor loses any heat to the surroundings. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A doubled styrofoam cup fitted. a calorimeter is a device used to determine heat flow during a chemical or. Calorimetry Lab Assumptions.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry Calorimetry Lab Assumptions a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. No calorimeter is perfect, however. For example, when an exothermic reaction. a perfect calorimeter absorbs no heat from the solution that it contains, nor loses any heat to the surroundings. the assumption behind the science of calorimetry is. Calorimetry Lab Assumptions.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimetry Lab Assumptions the assumption behind the science of calorimetry is that the energy gained or lost by the water is equal to the energy lost or gained by the object under study. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. a calorimeter. Calorimetry Lab Assumptions.
From www.studocu.com
Calorimetry Lab SE Lab General Physics 1 Studocu Calorimetry Lab Assumptions the assumption behind the science of calorimetry is that the energy gained or lost by the water is equal to the energy lost or gained by the object under study. A doubled styrofoam cup fitted. No calorimeter is perfect, however. a perfect calorimeter absorbs no heat from the solution that it contains, nor loses any heat to the. Calorimetry Lab Assumptions.